EARLY PREGNANCY LOSS
T. Flint Porter
D. Ware Branch
James R. Scott
(from Danforth's Obstetrics and Gynecology, 10th Edition, Editor : Gibbs, Ronald S.; Karlan, Beth Y.; Haney, Arthur F.; Nygaard, Ingrid E.)
Miscarriage, also termed spontaneous abortion, is most commonly used to describe first-trimester loss, although it has also been used to describe loss before 20 weeks. These arbitrary time limits have become less useful with advances in developmental biology and diagnostic sonography. Early pregnancy loss is more precisely defined as preembryonic (conception through the first 5 weeks of pregnancy from the first day of the last menstrual period), embryonic (6 to 9 weeks gestation), or fetal (10 weeks until delivery).
Epidemiology
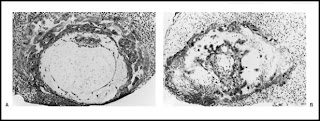
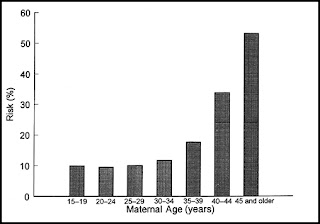
Miscarriage is the most common complication of pregnancy, occurring in at least 15% of clinically recognized pregnancies. Histologically defective ova found in hysterectomy specimens (Fig. 1) and data on early pregnancies detected with sensitive β-human chorionic gonadotropin (β-hCG) assays indicate that the rate is two to three times higher in early, unrecognized pregnancies. Miscarriage rates also vary with maternal age, ranging from 12% in women younger than 20 years of age to over 50% in women older than 45 years of age (Fig. 2). The likelihood of miscarriage is heavily dependent on past obstetric history, being higher among women with prior miscarriages and lower among women whose past pregnancy or pregnancies ended in live births.
Embryology
Successful pregnancy is dependent on integration of several complex processes involving genetic, hormonal, immunologic, and cellular events, all working together in perfect order to achieve fertilization, implantation, and embryonic development. It is not surprising that early pregnancy loss can occur because of a number of embryonic and parental factors.
Embryonic Factors
Most single, sporadic miscarriages are caused by nonrepetitive intrinsic defects in the developing conceptus, such as abnormal germ cells, chromosomal abnormalities in the conceptus, defective implantation, defects in the developing placenta or embryo, accidental injuries to the fetus, and probably other causes as yet unrecognized. Fifty percent of women presenting with spotting or cramping already have a nonviable conceptus by sonogram, and many of these embryos are morphologically abnormal. About one third of abortus specimens from losses occurring before 9 weeks gestation are anembryonic. Some cases of empty gestational sacs or “blighted ova” actually represent pregnancy failures with subsequent embryonic resorption. The high proportion of abnormal aborted concepti is apparently the result of a selective process that eliminates about 95% of morphologic and cytogenetic errors.
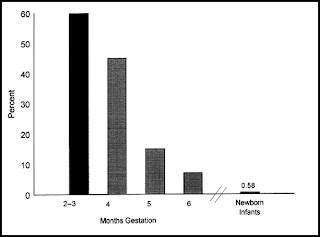
The frequency of chromosomally abnormal spontaneously aborted products of conception in the first trimester is approximately 60%, decreasing to 7% by the end of the 24th week (Fig. 3). The rate of genetic abnormalities is higher in anembryonic miscarriages. Autosomal trisomies are the most common (51.9%), arising de novo as a result of meiotic nondisjunction during gametogenesis in parents with normal karyotypes. The relative frequency of each type of trisomy differs considerably. Trisomy 16, which accounts for about one third of all trisomic abortions, has not been reported in live-born infants and is therefore uniformly lethal. Trisomy 22 and 21 follow in frequency. The next most common chromosomal abnormalities, in decreasing order, are monosomy 45,X (the single most common karyotypic abnormality), triploidy, tetraploidy, translocations, and mosaicism.
Media publicity tends to give the impression that a variety of agents such as infections, video display terminals, cigarette smoking, coffee, ethanol, chemical agents, and drugs markedly increase the risk of miscarriage. In reality, there is little credible supportive evidence.
Figure 1 Histologic comparison of (A) a morphologically normally implanted human ovum estimated to be about 11 to 12 days of age with (B) an abnormal conceptus, showing a defective trophoblast with pathologically large lacunae and an empty chorionic sac that is destined to abort. (From Hertig AT, Rock J,
Figure 2 Relation of maternal age to the risk of spontaneous abortion. (Data from Warburtin D, Kline J, Stein Z, et al. Cytogenetic abnormalities in spontaneous abortions of recognized conceptions. In: Porter IH, ed. Perinatal genetics: diagnosis and treatment.
Figure 3 The frequencies of chromosomal anomalies among 3040 spontaneously aborted fetuses related to the duration of pregnancy. For comparison, the frequency of chromosomal anomalies among 54,749 newborn infants is shown. (Data from Shiota K, Uwabe C, Nishimaura H. High prevalence of defective human embryos at the early implantation period. Teratology; Boue J, Boue A, Lazar P. Retrospective and prospective epidemiological studies of 1500 karyotyped spontaneous human abortions. Teratology 1975;12:11; Lauritsen JG. Aetiology of spontaneous abortion: a cytogenetic and epidemiological study of 288 abortuses and their parents. Acta Obstet Gynecol Scand Suppl 1976;52:1; and Creasy MR, Crolla JA, Alberman ED. A cytogenetic study of human spontaneous abortions using banding techniques. Hum Genet 1987;35:309 1976;31:177
Pathology
Most miscarriages occur within a few weeks after the death of the embryo or rudimentary analog. Initially, there is hemorrhage into the decidua basalis, with necrosis and inflammation in the region of implantation. The gestational sac is partially or entirely detached. Subsequent uterine contractions and dilation of the cervix eventually result in expulsion of most or all of the products of conception. When the sac is opened, fluid is often found surrounding a small macerated embryo, although no visible embryo may be present. Histologically, hydropic degeneration of the placental villi caused by retention of tissue fluid is common.
Clinical Features and Treatment
An unrecognized pregnancy should always be considered in any woman of reproductive age with abnormal bleeding or pain. Likewise, a patient with known pregnancy should notify her physician promptly about vaginal bleeding or uterine cramps. Since management depends on several clinical factors, it is useful to consider miscarriage under the following subgroups.
Threatened Miscarriage
Any bloody vaginal discharge or uterine bleeding that occurs during the first half of pregnancy has traditionally been assumed to be a threatened miscarriage. Spotting or bleeding during the early months of gestation occurs quite commonly, in as many as 25% of pregnant women. Bleeding is typically scanty, varying from a brownish discharge to bright red bleeding. It may occur repeatedly over the course of many days and usually precedes uterine cramping or low backache. On pelvic examination, the cervix is closed and uneffaced, and no tissue has passed. The differential diagnosis includes ectopic pregnancy, molar pregnancy, vaginal ulcerations, cervicitis with bleeding, cervical erosions, polyps, and carcinoma.
Women presenting with threatened miscarriage should receive an ultrasound examination to determine location, viability, and gestational age. Accurate knowledge of gestational age is necessary for proper interpretation, as a sonographically empty uterus may imply an abnormal intrauterine or ectopic pregnancy when it actually represents a normal early gestation. Serial testing of β-hCG measurements is a useful adjunct if the diagnosis remains uncertain, along with a follow-up sonogram a few days later.
A viable conceptus can be detected with modern ultrasound as early as 5.5 weeks gestation. It is possible to visualize the yolk sac and gestational sac starting at 5 to 6 weeks by using transvaginal ultrasound, with cardiac activity seen thereafter. Ultrasound findings suggesting impending pregnancy loss include an abnormally sized or shaped gestational sac and yolk sac, an embryo small for dates, and slow embryonic heart rate. In the absence of signs of miscarriage, more than 95% of pregnancies continue if a live embryo is demonstrated sonographically at 8 weeks gestation. Even in the setting of uterine bleeding, more than two thirds survive as long as ultrasound demonstrates an appropriately sized embryo with a normal cardiac rate. The subsequent pregnancy loss rate is only 1% if a live fetus is seen at 14 to 16 weeks gestation.
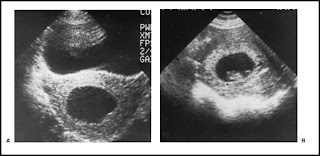
Although there is no convincing evidence that any treatment favorably influences the course of threatened miscarriage, a sympathetic attitude by the physician along with continuing support and follow-up are important to patients. This includes a tactful explanation about the pathologic process and favorable prognosis when the pregnancy is viable. An optimistic but cautious approach is prudent, since a few of these women will have a later embryonic or fetal death. It is reasonable to advise patients to remain available to medical care until it can be determined whether the symptoms will persist or cease. Continued observation is indicated as long as bleeding and cramping are mild, the cervix remains closed, quantitative β-hCG levels are increasing normally, and a normal embryo or fetus is evident on follow-up sonogram. If the bleeding and cramping progressively increase, the prognosis becomes worse. An unfavorable outcome is also associated with negative or falling β-hCG values, sonographic evidence of an embryo or fetus decreasing in size (Fig. 4), a slow heart rate, and a uterus that is not increasing in size on pelvic examination.
Inevitable and Incomplete Miscarriage
Miscarriage is considered inevitable when bleeding and cramping is accompanied by gross rupture of the membranes or cervical dilation. The miscarriage is incomplete when the products of conception have partially passed from the uterine cavity, are protruding from the external os, or are in the vagina with persistent bleeding and cramping. There is no viable conceptus in most instances of inevitable or incomplete miscarriages. Rarely, a single twin may survive and continue to term after miscarriage of the other conceptus.
Women with incomplete or inevitable miscarriage typically present with bleeding that can be profuse occasionally and produce hemodynamic instability. A careful pelvic examination is usually sufficient to establish the diagnosis, although ultrasound examination is often performed. Evacuation of the uterus is advisable to prevent further maternal hemorrhage or infection. Clinically stable patients can be treated as outpatients by either medical or surgical means. However, patients with uncontrolled bleeding should be transferred to the operating room for an examination under anesthesia and immediate surgical evacuation of the uterus. They should be observed postoperatively for several hours and discharged when considered stable.
Figure 4 Ultrasonic comparison of (A) an anembryonic pregnancy with no fetal tissue that is destined to abort with (B) a normal gestational sac with a transonic area, echogenic rim, and fetal pole.Suction curettage can be performed promptly and safely in an inpatient or outpatient setting by using analgesia, a paracervical block, and an intravenous infusion of normal saline containing 10 to 20 U of oxytocin. The cervix is sometimes dilated, and ring forceps can be used to remove products of conception from the cervical canal and lower uterine segment, thereby facilitating uterine contractions and hemostasis. Suction curettage with a plastic curette and vacuum pressure is used to remove the remaining tissue. The curette is rotated 360 degrees clockwise as it is withdrawn, and the procedure is repeated in a counterclockwise direction. When a grating sensation is noted and no more tissue is obtained, the endometrial cavity has been emptied. The tissue obtained should be examined to confirm the presence of products of conception and rule out the possibility of ectopic pregnancy.
Problems that can occur include allergic reactions to medication, uterine atony, uterine perforation, seizure, or cardiac arrest. A complete blood count level should be obtained, and blood replacement may be necessary if hemorrhage occurs. Rh-negative women should receive 50 g (in the first trimester) or the standard 300-g (in the second trimester) dose of Rh immune globulin to prevent Rh immunization.
Medical management of incomplete miscarriage has been studied in well-designed trials and may be used instead of surgical evacuation in clinically stable patients, although uterine curettage may eventually be required. In one randomized, controlled trial, an 80% complete abortion rate was achieved by using 800 mg of misoprostol (four 200-mg tablets) per vagina every 4 hours. Most patients responded to the first dose. Curettage was necessary in 28% of patients. When successful, misoprostol treatment of incomplete miscarriage is associated with lower rates of short- and long-term complications compared with surgical evacuation. Combinations of misoprostol with methotrexate or RU-486 appear promising but are not available for clinical use.
Complete Miscarriage
Patients followed for a threatened miscarriage should be instructed to save all tissue passed for later inspection. When the entire products of conception have passed, pain and bleeding soon cease. If the diagnosis is certain, no further therapy is necessary. In questionable cases, ultrasound is useful to confirm an empty uterus. In some cases, curettage may be necessary to be sure that the uterus is completely evacuated. Removal of remaining necrotic decidua decreases the incidence of bleeding and shortens the recovery time.
Missed Miscarriage
The reason that expulsion of a dead conceptus does not occur despite a prolonged period is uncertain. The patient's symptoms of pregnancy typically regress, quantitative β-hCG levels fall, and no fetal heart motion is detected by ultrasound. While most patients eventually abort spontaneously, and coagulation defects due to retention of the conceptus are rare, expectant management is emotionally trying, and many women prefer to have the uterus evacuated. Either medical or surgical evacuation of uterine contents is acceptable.
In the second trimester, the uterus can be emptied by dilation and evacuation (D&E) or induction of labor with intravaginal prostaglandin E2 (PGE2) or misoprostol. D&E is an extension of the traditional dilation and curettage (D&C) and vacuum curettage. It is especially appropriate at 13 to 16 weeks gestation, although many proponents use this procedure through 20 weeks. The cervix is usually first prepared by using misoprostol or passively dilated with laminaria to avoid trauma, and the fetus and placenta are mechanically removed with suction and instruments.
If induction of labor is chosen, vaginal PGE2 may be used; one 20-mg suppository is placed high in the posterior vaginal vault every 4 hours until the fetus and placenta are expelled. Between 2.5 and 5.0 mg of diphenoxylate given orally and 10 mg of prochlorperazine given intramuscularly can control diarrhea and nausea, and narcotics or epidural anesthesia can be used to control pain. In this situation, a retained placenta is relatively common and may require manual removal and uterine curettage.
Misoprostol has become more commonly used in recent years because of its equal efficacy and markedly lower incidence of unpleasant side effects; 200-mg tablets are placed high in the vagina every 4 hours until delivery of the fetus and placenta. Medical treatment of nausea, vomiting, diarrhea, and fever are rarely necessary, although retained placenta is not uncommon.
Septic Miscarriage
Septic abortion, once a leading cause of maternal mortality, has become less frequent, primarily because of changes in abortion laws making pregnancy terminations more easily available to women with unwanted pregnancies. However, any type of spontaneous miscarriage can also be complicated by endometritis, which can progress to parametritis and peritonitis. The clinical presentation typically includes fever, abdominal tenderness, and uterine pain.
Septicemia and shock may occur if the local infection is left untreated. The polymicrobial infection mirrors the endogenous vaginal flora and includes Escherichia coli and other aerobic, enteric, gram-negative rods, group B-hemolytic streptococci, anaerobic streptococci, Bacteroides species, staphylococci, and microaerophilic bacteria.
The initial evaluation and management of septic abortion should include:
- Physical and pelvic examination
- Complete blood cell count and determination of electrolyte, blood urea nitrogen, and creatinine levels
- Type and screen or cross match of blood
- Smears from cervix for Gram stain
- Aerobic and anaerobic cultures of endocervix, blood, and available products of conception
- Placement of indwelling Foley catheter
- Administration of intravenous fluids (e.g., saline, Ringer lactate) through a large-bore angiocatheter
- Administration of 0.5 mL of tetanus toxoid, given subcutaneously for immunized patients, or 250 U of tetanus immune globulin, administered deep within the muscle
- Abdominal x-rays to detect free air or foreign bodies.
| TABLE 1 Antibiotic Regimens for Septic Abortion | ||||
|
Recurrent Miscarriage
Recurrent miscarriage (RM), traditionally defined as three or more consecutive first-trimester spontaneous losses, affects up to 1% of couples. Primary RM is diagnosed in women without any prior successful pregnancy, while secondary RM refers to those whose repetitive losses follow a live birth. There is no specific classification for women who have multiple miscarriages interspersed with normal pregnancies. It is generally agreed that a workup for possible causes of RM is indicated in most patients after two or three consecutive miscarriages.
The evaluation and subsequent management of couples with RM is controversial, receiving considerable attention in the lay and medical literature in recent years. General etiologic categories of RM include genetic, uterine pathologic, endocrine, immunologic, thrombophilic, and environmental. Unfortunately, a cause for recurrent pregnancy loss is identified in only about 50% of affected couples. There is little evidence supporting poor nutrition, infections, unrecognized diabetes, toxic agents, or psychologic trauma as significant etiologic factors.
A typical evaluation of patients with RM includes investigation of anatomic, immunologic, endocrine, genetic, and infectious factors. These may be criticized because the derivation of their diagnostic use and treatments advocated are empirical, and many come under scrutiny because they were never submitted to a properly designed study. Importantly, evidence has mounted that the average woman with RM has a fairly good prognosis for a successful next pregnancy without any specific treatment.
Some alleged causes of RM have received considerable attention, and new diagnostic tests for RM are continually being proposed to replace those that have been previously disproved and discarded. Among these are antithyroid antibodies, elevated follicular-phase luteinizing hormone levels, circulating maternal embryotoxic factor, and abnormal lymphocyte subset ratios (elevated CD56+ levels). Most concerning is that despite a lack of scientific supportive evidence, empiric and alternative treatment regimens based on these tests are prescribed to women who are desperate to seek a solution to their repeated losses. Moreover, it is beyond the scope of this chapter to critically analyze each new treatment or assay, but the mechanism of pregnancy loss and potential relationship to each of these remains largely theoretical. Until effective treatments are identified and proven by properly designed studies, these screening tests have little use in the routine evaluation of patients with RM.
Known and Suspected Causes of Recurrent Miscarriage
Structural Uterine Defects
The mechanism of pregnancy loss in women with uterine anomalies is uncertain, although a diminished blood supply interfering with normal implantation and placentation and the reduced size of the uterine cavity are often cited as possible culprits. Between 7% and 8% of women have a uterine abnormality (mallerian anomaly), with the prevalence among women with RM estimated to be 10% to 15%. The prognosis for successful pregnancy is related to the type of malformation, with asymmetric fusion defects carrying the worst prognosis and septate, bicornuate, and didelphic uteri carrying increasingly better prognoses. While an arcuate uterus is the most commonly identified mallerian anomaly, its association with adverse reproductive outcome, including RM, is uncertain. Other uterine abnormalities such as leiomyoma, diethylstilbestrol (DES) exposure, and intrauterine synechiae (Asherman syndrome) may interfere with implantation and also result in pregnancy loss.
Hysterosalpingography, magnetic resonance imaging (MRI), hysteroscopy, sonohysteroscopy, and laparoscopy can be used to diagnose uterine structural defects. Abdominal metroplasty has been replaced in most cases by the hysteroscopic removal of uterine septa. Hysteroscopy can be accomplished in an outpatient setting and eliminates the need for cesarean delivery in patients who achieve pregnancy. Uncontrolled, retrospective studies suggest that the subsequent live-birth rate is greater than 80%. Removal of synechiae and submucous myomas can also be performed hysteroscopically.
Endocrine Dysfunction
The luteal phase defect (LPD) has long been suspected of causing sporadic miscarriage. Evidence linking LPD to RM is less certain and subject to criticism. Women with LPD are thought to have short menstrual cycles, postovulatory intervals less than 14 days, and secondary infertility. Initially, LPD was theorized to result from a failure of the corpus luteum to make enough progesterone to establish a mature endometrial lining suitable for placentation. This theory has evolved to implicate poor follicular-phase oocyte development, which also results in disordered estrogen secretion, inadequate ovarian steroidogenesis, and subsequent maldevelopment of endometrial receptors. In turn, these effects could result from excess luteinizing hormone or hyperandrogenic states.
Some investigators claim that LPD accounts for over one fourth of cases of RM, but none of their studies has included concurrently tested controls, and they cannot agree on appropriate diagnostic criteria. Originally, LPD was diagnosed in the presence of so-called “out-of-phase” endometrial biopsies, which lagged 2 days behind the actual ovulation date, estimated by counting backward from the next menstrual period. However, the diagnostic accuracy of endometrial tissue phasing fell out of favor because of considerable interobserver and intraobserver variation in pathologic interpretation, not to mention the fact that many women without miscarriage exhibit the same out-of-phase endometrial biopsies. Progesterone levels have also been proposed as diagnostic criteria for LPD. However, in patients with RM, low serum progesterone in the luteal phase is only 71% predictive of LPD based on an abnormal endometrial biopsy.
Despite this, endometrial biopsy or luteal-phase serum progesterone levels are widely used to make the diagnosis of LPD. In turn, many clinicians treat women with supposed LPD and RM with progesterone in a subsequent pregnancy. One commonly advocated treatment is a 25-mg progesterone suppository, administered vaginally twice daily (morning and night) with treatment beginning after ovulation and continuing until either menses begin or through the first 8 to 10 weeks of pregnancy. Comparable doses of oral micronized progesterone have also been used. Early studies of progesterone supplementation reported improved pregnancy outcomes in treated women. However, the reliability of these findings has been questioned because no appropriate control groups were treated for comparison. The most recent meta-analysis of progesterone for women with RM found no benefit in the prevention of miscarriage.
Clomiphene and other ovulatory agents, as well as human chorionic gonadotropin (hCG), have also been tried in an attempt to improve follicular development and stimulate corpus luteum function in women with LPD, with varying results. One placebo-controlled, multicentered trial using hCG found no significant difference in the successful pregnancy rates (83% vs. 79%).
Polycystic Ovarian Syndrome
A possible link between polycystic ovarian syndrome (PCOS) and RM has been hypothesized based on the finding that 36% to 56% of women with RM have PCOS based on ultrasound examination of the ovaries. Interestingly, sonographic evidence of PCOS in women with RM does not predict miscarriage when compared with women with RM without PCOS. It has been postulated that pregnancy loss in women with PCOS may be related to elevated serum utilizing hormone levels, high testosterone and androstenedione concentrations, and/or insulin resistance. Indeed, insulin resistance is more common in women with RM compared with fertile controls regardless of whether or not they have PCOS. Treatment of insulin resistance with metformin has been reported to reduce miscarriage in small studies. However, none has included appropriate control groups, and study subjects have not necessarily had RM. Metformin has been shown to cross the placenta, although several studies have failed to find evidence of teratogenesis when used during pregnancy.
Genetic Abnormalities
Approximately 2% to 4% of couples experience RPL because one partner is a carrier of a balanced structural chromosomal rearrangement, usually a balanced translocation. The incidence of balanced translocations is twofold higher among females compared with males. While carriers of balanced translocations are phenotypically normal, meiotic segregation results in chromosomal duplications or deficiencies in offspring leading to spontaneous abortion or an abnormal live born. About 60% of balanced translocations are reciprocal, while 40% are robertsonian. The risk of recurrent aneuploidy is dependent on which parent is heterozygous for the translocation as well as the chromosomes involved. In general, the risk is higher if the translocation is maternal in origin; translocations involving homologous chromosomes preclude the possibility of any normal live-born infants.
Chromosomal inversions have also been linked to RPL. The risk of abnormal offspring depends on the size and location of the inversion and whether or not the carrier is male or female. Inversions of small portions of the total chromosomal length lead to large duplications and deficiencies and are generally lethal. Paradoxically, larger inversions are more likely to be compatible with survival. The risk of abnormal offspring is slightly higher if the heterozygous carrier of a pericentric inversion is female (7% vs. 5%). Paracentric recombinants are universally lethal.
The evaluation of couples with RM should include cytogenetic evaluation of both partners. Genetic counseling should be given to those with parental chromosomal abnormalities in an effort to predict recurrence, and genetic amniocentesis or chorionic villus sampling should be offered in subsequent pregnancies. Chromosomal analysis of the products of conception is also clinically useful, particularly in the evaluation of the reason for failure of a treatment regimen. Parental chromosomal abnormalities do not usually preclude further attempts at pregnancy, because most couples eventually have normal offspring. For the rare homologous robertsonian translocation that prevents successful pregnancy, therapeutic possibilities include artificial donor insemination, in vitro fertilization with donor oocytes, and adoption.
Molecular mutations that may be shown in the future to cause recurrent miscarriages include lethal, single-point mutations, possibly linked to MHC genes; mutations in genes that code for products critical for normal development; mutations in homeobox genes that control transcriptional regulation; mutations that lead to severe metabolic errors and embryonic death; and disorders of protooncogenes and oncogenes. One group has shown that certain polymorphisms of the HLA-G gene are associated with significantly higher rates of miscarriage among couples presenting with RM. Marked skewing of the normal 50:50 distribution of X chromosome inactivation in the mother, a condition termed highly skewed X-chromosome inactivation, has been theorized to cause otherwise unexplained RM. However, recent studies have found no association between RM and skewed X chromosome inactivation.
Antiphospholipid Syndrome and Other Autoimmune Disorders
Antiphospholipid syndrome (APS) has been recognized as a proven cause of pregnancy loss in approximately 5% to 15% of women with RM. The diagnosis is based on the presence of either the lupus anticoagulant (LA), moderate to high levels of IgG anticardiolipin (aCL) antibodies, or both. These acquired antiphospholipid autoantibodies are induced by as yet unknown stimuli in the setting of aberrant immunoregulation. Low levels of immune globulin G or IgM aCL are of questionable significance.
Although women with APS may present with RM in the first trimester, fetal death in the second or early third trimesters may be more specific for the condition. Patients with high levels of IgG aCL or a history of prior fetal death are at greatest risk of another fetal loss. The cause of fetal death appears to be a decidual vasculopathy that results in decidual infarction and insufficient blood flow to the placenta. Intervillous thrombosis has also been described. However, these lesions are nonspecific, and the degree of pathology is not always sufficient to explain the fetal death. The mechanisms by which aCL may cause decidual vasculopathy and fetal death are unknown. A number of pathophysiologic mechanisms have been proposed, including an imbalance of local prostacyclin and thromboxane production, enhanced platelet aggregation, decreased activation of protein C, increased tissue factor, and decreased trophoblast annexin V production or availability. Most recently, the complement system has been invoked as having a major role in APS-related pregnancy loss.
Maternally administered unfractionated heparin (UF) and low-molecular-weight heparin (LMWH) are considered the treatment of choice for APS pregnancies, both to improve embryo-fetal outcome and to protect the mother from thrombotic events (Table 2). Treatment regimens are usually initiated in the early first trimester after ultrasonographic demonstration of an intrauterine pregnancy. The optimal dosing regimen is debated. Some experts advocate thromboprophylactic, unadjusted, low doses of UF (e.g., 5,000 to 7,500 U b.i.d.) or once daily LMWH (1 mg/kg) when treating women with APS and a history of RM and other APS-related complications in the absence of prior thrombosis. However, full-dose anticoagulation regimens with UF or LMWH (1 mg/kg b.i.d.) are generally recommended for pregnant APS patients with prior thrombosis. In most case series and trials, daily low-dose aspirin is also included in the treatment regimen. One important caveat deserves mention a small, placebo-controlled trial found that otherwise healthy women with RM and low titers of antiphospholipid antibodies do not require treatment.
Intravenous immune globulin (IVIG) has also been used during pregnancy, usually in conjunction with heparin and low-dose aspirin, especially in women with APS and particularly poor past histories or RM during heparin treatment. However, a randomized, controlled, pilot study of IVIG treatment during pregnancy in unselected APS cases proved negative.
Anticoagulant coverage of the postpartum period in women with APS, regardless of prior thrombosis history, is critical. Heparin regimens may be continued, or patients can be transitioned to warfarin thromboprophylaxis after delivery. In most cases, an international normalized ratio of 3.0 is desirable, and postpartum coverage should extend for 6 to 8 weeks after delivery. Both heparin and warfarin are safe for nursing mothers. The need for postpartum anticoagulation in women with primary APS diagnosed solely on the basis of recurrent preembryonic and embryonic losses is unclear.
Autoantibodies to thyroid antigens are associated with a modest increased rate of pregnancy loss if identified in early pregnancy or immediately before pregnancy. Some investigators have found a significant proportion of women with RM to have antithyroid antibodies; others have not. Even so, no treatment options for women with RM and antithyroid antibodies have proven beneficial.
Approximately 15% of women with RM have detectable antinuclear antibodies (ANA). However, subsequent pregnancy outcomes among women with a positive ANA test result are no different from those among women with a negative ANA test result. A randomized treatment trial of women with RM and a positive ANA found no benefit to treatment with prednisone and low-dose aspirin compared with treatment with placebo. Currently available data do not support testing women with RM for ANA.
Thrombophilic Disorders
Inherited thrombophilic disorders are identified in 50% of women with pregnancy-related venous thrombosis as well as in women with several obstetric complications including RPL, preeclampsia, and uteroplacental insufficiency. Thrombophilic defects, including factor V Leiden and prothrombin G20210A mutations and deficiencies in protein C, protein S, and antithrombin III, have been reported significantly more often in women with pregnancy complications compared with women with normal pregnancies. The factor V Leiden and prothrombin G20210A mutations are by far the most common of the inherited thrombophilias, present in 8% and 3%, respectively, of the general white population in the
The high prevalence of inherited thrombophilias in women with obstetric complications has led to the use of prophylactic anticoagulation during pregnancy. However, there is limited supportive evidence for this practice in women without a history of venous thrombosis. A variety of treatment regimens have been used, and no study has included an appropriate control group for comparison. Until randomized controlled trials have been performed, prophylactic anticoagulation should be reserved for selected women with thrombophilia and RM after an informed discussion of the risks and limited data suggesting benefit.
Idiopathic Recurrent Miscarriage
An etiology cannot be identified in at least 50% of couples with RM, despite a thorough evaluation. This has led to speculation about other potential causes of RM with particular attention to the role of alloimmune factors in pregnancy maintenance. A relationship between alloimmunity and RM has yet to be proven, largely because little is known about the mechanisms that prevent immunologic rejection of the conceptus in successful pregnancies. Early reports proposed that HLA compatibility between couples, the absence of maternal leukocytotoxic antibodies, or the absence of maternal blocking antibodies were related to RM. The importance of these factors has not been substantiated, and tests for detection are expensive and not clinically useful. Research has also focused on local decidual or trophoblast immunosuppressive factors such as cytokines, growth factors, hormones, enzymes, and endometrial proteins. Some of these immunoactive factors appear to be necessary for implantation and growth and development of the early placenta and embryo, and others may cause abortion, when expressed. There are, however, no practical clinical tests available for these factors and no proven treatment if they were found abnormal.
Although no alloimmune mechanism has been unequivocally shown to cause RM in humans, several types of immunotherapy have been advocated. Originally, the attempt to improve maternal immunotolerance in recurrent aborters was based on evidence that pretransplant blood transfusions decreased rejection of organ allografts and that the rate of resorption or abortion in animal models was reduced by prior immunization with spleen cells from a paternally related strain. The most popular regimen involves injections of the father's leukocytes. Although proponents persist, this treatment is questionable at best and harmful at worst. Most randomized trials have proven negative, and the largest and only multicenter randomized trial found that treated pregnancy outcomes were worse in the women who received leukocyte immunization. Based largely on this trial, the U.S. Food and Drug Administration has stated that the administration of this therapy for RM may only be done as part of a clinical investigation, and then only if there is an investigational new drug application in effect. Participating women should be counseled that immunization using viable leukocytes carries the risks of any blood transfusion, such as hepatitis, HIV, and cytomegalovirus infections. Reactions have been uncommon but include soreness and redness at the injection site, cutaneous graft-versus-hostlike reaction, fever, maternal platelet and leukocyte alloimmunization, and blood group sensitization.
Intravenous immune globulin has been proposed as an alternative therapy in patients with idiopathic RM. A number of randomized trials have been reported, and the results are conflicting. Nevertheless, this treatment seems to be no more successful than paternal cell immunization, and IVIG is not recommended outside of a research protocol by either the ACOG or the American Society of Reproductive Medicine.
It is imperative for physicians to recognize that the prognosis for idiopathic RM is by no means dismal. Numerous studies and several meta-analyses indicate that the average next pregnancy live-birth rate for placebo-treated women with idiopathic RM is 60% to 70%. Many couples see this modestly favorable prognosis in a somewhat positive light, and it compares favorably with the prognosis for conditions such as APS or parental karyotype abnormalities. Understanding this prognosis may allow the couple to choose against an expensive unproven treatment. One caveat-as expected, increasing maternal age and increasing number of miscarriages are negative variables.
Recommendations for Recurrent Miscarriage
The scheme for a reasonable and cost-effective evaluation of women with RM shown in Table 3 is based on current guidelines published by the ACOG and the Royal College of Obstetricians and Gynaecologists. A sympathetic attitude by the physician is crucial-establishment of trust and rapport and a sincere appreciation of the distress and grief experienced by these couples permit tactful and thorough discussions with patient and partner. It is reasonable to institute an evaluation after two consecutive miscarriages in anxious women or if the patient has few reproductive years remaining or has had an infertility problem. Couples interested in an investigational protocol are perhaps best referred to legitimate research centers.
| TABLE 3 Suggested Evaluation of Patients with Recurrent Miscarriage | ||||
|
SUMMARY
- Miscarriage occurs in at least 15% of clinically recognized pregnancies; the rate is two to three times higher in early, unrecognized pregnancies.
- More than 95% of pregnancies continue if a live embryo is demonstrated sonographically at 8 weeks gestation.
- Women with three or more consecutive miscarriages should undergo evaluation; workup may be appropriately sooner in older couples or in those with infertility.
- Miscarriage is the most common complication of pregnancy, and the most frequent etiology is a chromosomal abnormality of the conceptus.
- Ultrasound is helpful in determining whether or not the embryo is viable, and appropriate modern management may be observation or medical or surgical evacuation of the uterus.
- Misoprostol and curettage are equally safe and effective methods of uterine evacuation in patients with incomplete miscarriage.
- Recurrent early pregnancy loss is sometimes associated with underlying maternal abnormalities that can be detected with a standard evaluation.
- Unproven tests and controversial treatments for recurrent miscarriage should not be used routinely until supportive evidence is available.



1 comments:
I was diagnosed as HEPATITIS B carrier in 2013 with fibrosis of the
liver already present. I started on antiviral medications which
reduced the viral load initially. After a couple of years the virus
became resistant. I started on HEPATITIS B Herbal treatment from
ULTIMATE LIFE CLINIC (www.ultimatelifeclinic.com) in March, 2020. Their
treatment totally reversed the virus. I did another blood test after
the 6 months long treatment and tested negative to the virus. Amazing
treatment! This treatment is a breakthrough for all HBV carriers.
Post a Comment